Deviation from your prescribed flow could lead to increase in possible for microbial contamination. Substance/personnel flow might be modified, but the results on the changes from a microbiological point of view must be assessed by dependable administrators and need to be licensed and documented.
FARRAR® has two different techniques to working experience our products and solutions. At our headquarters in Davidson, NC, our BioSolutions Area includes fully operational ULC models with standard content dealing with alternatives - feel free to go to this space to system your project and get the job done with our design staff over a personalized material dealing with Answer that fits your procedure.
The criticality of the amount of nonviable particulates from the Digital industry tends to make the appliance of Federal Standard 209E a requirement, although the pharmaceutical industry provides a bigger worry for feasible particulates (i.
Our engineered alternatives are perfect for companies dedicated to mission-significant refrigeration procedures and storage. FARRAR chambers adhere to restricted environmental technical specs that will help shield concluded goods, bulk unfinished merchandise, frozen biologic material, and also vaccine and drug substances.
Administration of the power should guarantee that all personnel associated with operations in clean rooms and managed environments are very well versed in related microbiological principles. The teaching should really include instruction on The essential ideas of aseptic processing and the relationship of manufacturing and managing treatments to likely sources of merchandise contamination. This teaching need to include things like instruction on the basic concepts of microbiology, microbial physiology, disinfection check here and sanitation, media assortment and preparation, taxonomy, and sterilization as required by the character of staff involvement in aseptic processing.
The ULC has extreme cooling capability to freeze products. The duration of the freeze method for that Extremely-Reduced Chamber (ULC) Series will range relying the quantity of fabric to freeze, plus the commencing and intended ending temperature of the material.
Padded mats and cooler temperatures in labs in which personnel have to have on coats, gloves, and hats also boost ease and comfort for a far better Doing the job natural environment.
Following the investigation, actions taken may perhaps include reinforcement of training of personnel to emphasise the microbial Charge of the natural environment; added sampling at greater frequency; additional sanitization; further item testing; identification on the microbial contaminant and its doable supply; and an analysis of the necessity to reassess the current conventional working strategies also to revalidate them, if necessary.
To allow communication involving the remarkably controlled labs as well as the read more broader function spot of your pharmacy, the USP 797 and USP 800 rooms Each individual Have got a glass front and cell phone to take care of visual and auditory interaction in between the Areas.
Staff teaching is an important component of protecting sterility in pharmaceutical cleanrooms. As cleanroom technological know-how and sterilization practices evolve, so much too have to the techniques to coaching cleanroom staff.
The Ultra-Lower Chamber (ULC) Series has the opportunity to freeze merchandise from ambient temperatures to -eighty°C, but it does not make it possible for for exact cooling profiles for example fall and maintain or managed temperature alterations/moment.
Different phases of pharmaceutical manufacturing involve distinctive cleanroom classifications according to the extent of cleanliness expected. The most typical cleanroom classifications used in the pharmaceutical industry consist of:
Isolator devices need comparatively rare microbiological checking. Constant complete particulate monitoring can offer assurance which the air filtration procedure inside the isolator is Performing thoroughly. The solutions for quantitative microbiological air sampling explained Within this chapter might not have ample sensitivity to test the setting inside an isolator.
On the other hand, it is actually identified that repeated media runs are essential in order to affirm the statistical validity on the observed contamination rate for the process.
 Tahj Mowry Then & Now!
Tahj Mowry Then & Now!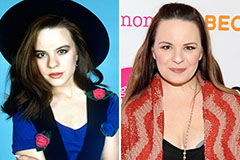 Jenna Von Oy Then & Now!
Jenna Von Oy Then & Now! Keshia Knight Pulliam Then & Now!
Keshia Knight Pulliam Then & Now! Hailie Jade Scott Mathers Then & Now!
Hailie Jade Scott Mathers Then & Now! McKayla Maroney Then & Now!
McKayla Maroney Then & Now!